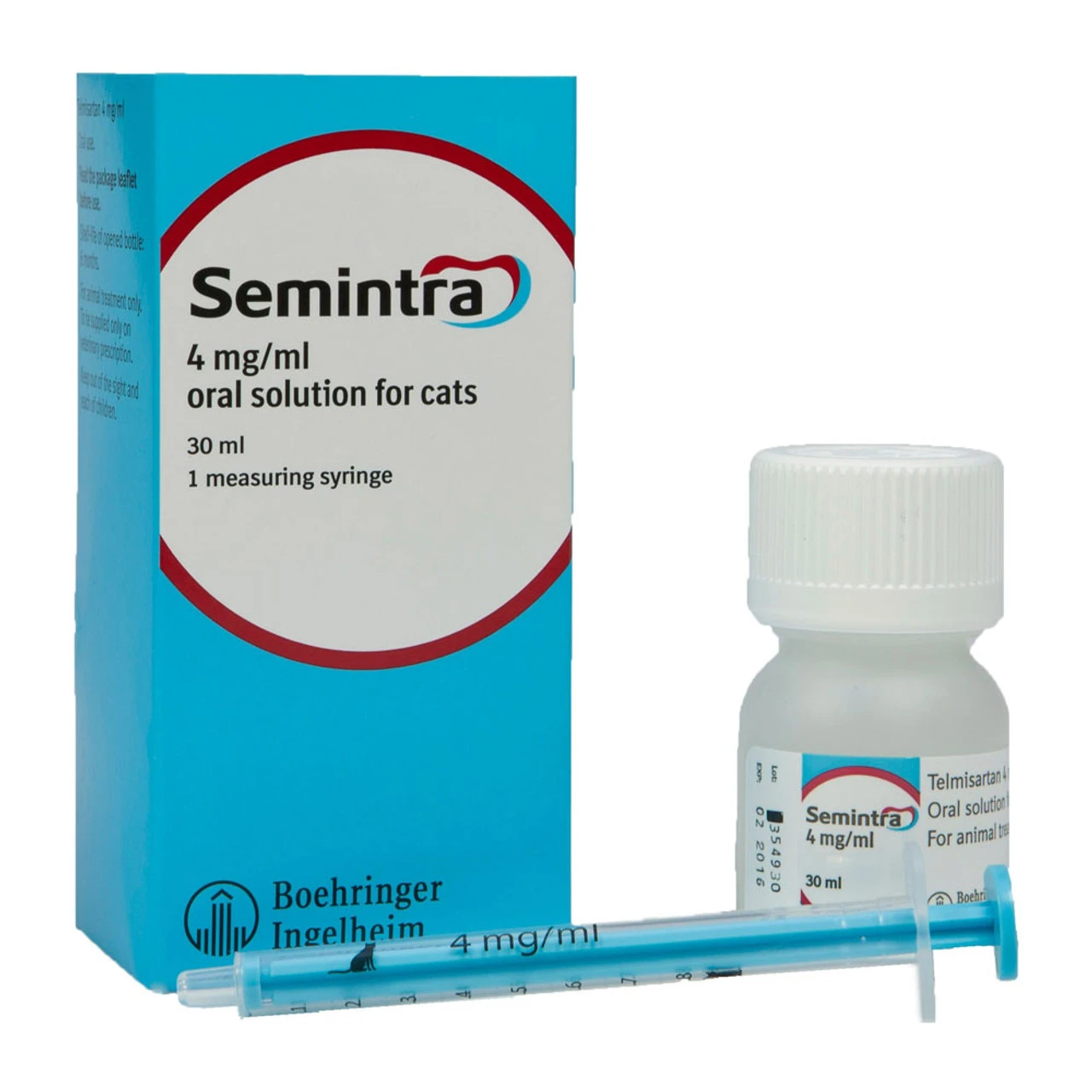
Semintra 4 Mg/ml Oral Solution For Cats
$24.82
- High quality products, low prices.
- Secure Shopping Starts with Safe Payments
- Buy quality, buy with us.
- Multiple safe payment methods

Semintra 4mg/ml Oral Solution for Cats.
Uses:
Reduction of proteinuria associated with chronic kidney disease (CKD) in cats.
Dosage and administration:
Oral use.
The recommended dose is 1 mg telmisartan/kg body weight (0.25 ml/kg body weight). The product is to be administered once daily directly into the mouth or with a small amount of food.
Semintra is an oral solution and is well accepted by most cats.
The solution should be given using the measuring syringe provided in the package. The syringe fits onto the bottle and has a kg-body weight scale.
After administration of the veterinary medicinal product close the bottle tightly with the cap, wash the measuring syringe with water and let it dry. To avoid contamination, use the provided syringe only to administer Semintra.
Contra-indications, warnings, etc:
Do not use during pregnancy or lactation. The safety of Semintra has not been established in breeding, pregnant or lactating cats.
Do not use in case of hypersensitivity to the active substance or to any of the excipients.
Special precautions for use in animals:
The safety and efficacy of telmisartan has not been tested in cats under the age of 6 months. It is good clinical practice to monitor the blood pressure of cats receiving Semintra which are under anaesthesia.
Due to the mode of action of the veterinary medicinal product, transient hypotension may occur. Symptomatic treatment, e.g. fluid therapy, should be provided in case of any clinical signs of hypotension.
As known from substances acting on the Renin-Angiotensin-Aldosterone System (RAAS), a slight decrease in red blood cell count may occur. Red blood cell count should be monitored during therapy.
The following mild and transient gastrointestinal signs have rarely been observed in a clinical study (in order of decreasing frequency): mild and intermittent regurgitation, vomiting, diarrhoea or soft faeces.
Elevated liver enzymes have been very rarely observed and values normalised within a few days following cessation of therapy.
Effects attributable to the pharmacological activity of the product observed at the recommended treatment dose included reductions in blood pressure and decreases in red blood cell counts.
During concomitant therapy with amlodipine at the recommended dose no clinical evidence of hypotension was observed.
After administration of up to 5-fold of the recommended dose for 6 months, no adverse reactions were observed other than those mentioned above.
Administration of the product at overdose (up to 5-fold of the recommended dose for 6 months) resulted in marked reductions in blood pressure, decreases in red blood cell count (effects attributable to the pharmacological activity of the product) and increases in Blood Urea Nitrogen (BUN). These effects are unlikely to be observed under clinical conditions. However, in the event that transient hypotension does occur, symptomatic treatment, e.g. fluid therapy, should be provided.
In the absence of compatibility studies, this veterinary medicinal product must not be mixed with other veterinary products.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
In case of accidental ingestion, seek medical advice immediately and show the package leaflet or the label to the physician.
Avoid eye contact. In case of accidental eye contact, rinse eyes with water. Wash hands after use.
Pregnant women should take special care to avoid contact with the product because substances acting on the RAAS, such as Angiotensin Receptor Blockers (ARBs) and ACE inhibitors (ACEi), have been found to affect the unborn child during pregnancy in humans.
People with hypersensitivity to telmisartan or other sartans/ARBs should avoid contact with the veterinary medicinal product.
Pharmaceutical precautions:
Shelf life of the veterinary medicinal product as packaged for sale (30 ml or 100 ml): 3 years.
Shelf life after first opening the immediate packaging: 6 months.
For animal treatment only. To be supplied only on veterinary prescription.
Keep out of the sight and reach of children.
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.
Legal category:
Legal category: POM-V
Packaging quantities:
Cardboard box containing one 45 ml HDPE bottle filled with 30 ml closed with a LDPE plug-in adapter and a tamper-proof child resistant closure and a measuring syringe. Cardboard box containing one 124 ml HDPE bottle filled with 100 ml closed with a LDPE plug-in adapter and a tamper-proof child resistant closure and a measuring syringe. Not all pack sizes may be marketed.
| Option | 100ml, 30ml |
|---|
Be the first to review “Semintra 4 Mg/ml Oral Solution For Cats” Cancel reply
Related products
Cat care
Cat care
Cat care
Cat care








Reviews
There are no reviews yet.